Products


VertehighFix High Viscosity Spinal Bone Cement System
VertehighFix is indicated for the fixation of pathological fractures of bone using vertebroplasty and kyphoplasty. Painful vertebral compression fractures of the vertebral body may result from osteoporosis, benign lesions (hemangioma), or malignant lesions (metastatic cancers, myeloma).
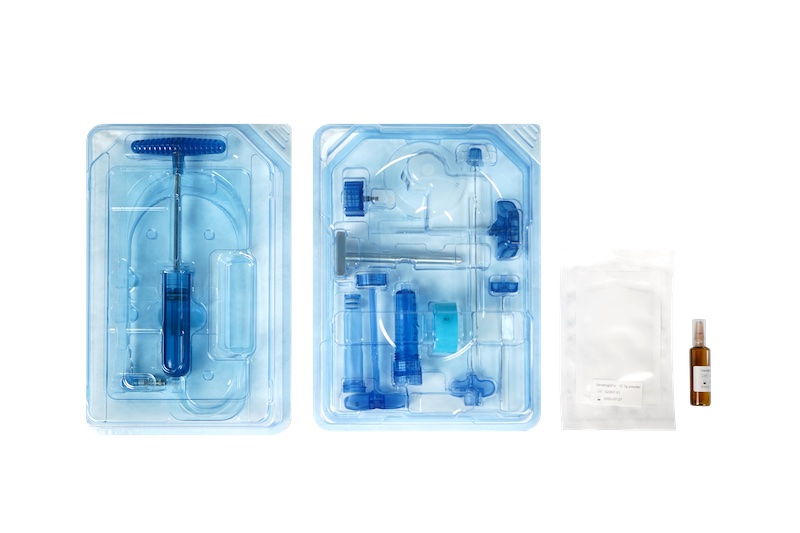
The product is divided two parts, bone cement and an injection tool.
- Bone Cement:“VertehighFix” High Viscosity Spinal Bone Cement (TFDA No.006941)
Composition:The product contains a three-layer bag of powder polymer and a blister liquid ampoule of MMA.
REF | Powder | Liquid |
HSA0125 | 12.5 g | 5 mL |
- Injection tool:Bone cement mixing device and delivery system
The bone cement mixing device can blend the powder and liquid, transferring the mixture to the injection barrel. Subsequently, the injection port connects to the introducer needle, and the bone cement is extruded and injected into the vertebral body by the hydraulic pump.
The delivery system is divided three-parts:
- Hydraulic pump (ISD0160):Manual hydraulic pump
- The bone cement mixing device includes:two Funnels, a mixing barrel, barrel holder, mixing cap, mixing rod, adapter ring, cement syringe, transfer rod, and cement syringe cap
- Introducer needles:Introducer needle (Double-diamond, ISF0110, ISF0111), Introducer needle, Side-Firing (ISH0130, ISH0131), Biopsy needle (ISI0150), and Guide needle (ISJ0001)
VertehighFix should be stored in its original, unopened packaging, in a dry, clean place. Keep away from light and at a temperature of 25℃ maximum.
For controlled and optimal use of VertehighFix, the doses are to be stored at the intended operating room temperature for a minimum of 24 hours before use.
SPECIFICATION
Name & Spec. | Bone Cement | Introducer Needle Kit (Blister packaging) | ||
VertehighFix High Viscosity Spinal Bone Cement | Hydraulic Pump | Bone Cement Mixing Device | ||
Model of Parts | HSA0125 | ISD0160 | ISC0200 | |
REF | HSA0100 | ◎ | ◎ | ◎ |